Problem
Starting a new job is always an exciting proposition, especially when you’ve been hired to create a brand new, global Quality Organization! You’ll probably start out with:
- A decentralized organization
- A blank process and procedure canvas
- No staff
- A budget torn from the company’s many operating divisions.
Where do you begin? Well, if you’re like one of our favorite clients, one of your 1st calls will be to Sandalwood!
A hallmark of a great leader is his/her ability to effectively communicate significant changes in policies and standards of governance to:
- Employees
- Service partners
- The global supply base
Insuring a smooth implementation of these polices is a cornerstone strategy to improving the capability of its supply base as well as reducing costs and supply chain risk. Sandalwood was given the task of creating and implementing such a communication mechanism: A Global Supplier Quality Manual.
Premise
The Global Supplier Quality Manual was collaboratively designed to address the following business methods and processes:
- New Product and Process Development
- Process Validation and Capability
- Capacity Planning and Delivery
- Preventive/Corrective Actions
- Change Management
- Supplier Assessments
- Key Process Indicators (KPi’s)
Challenges
Although the challenges were many, the combined Client and Sandalwood team successfully addressed the following:
- Decentralized internal organization
- Internal resources residing in multiple regions
- Lack of global standards
- Large global supply base – five regions (NA, South America, EU, MEA, and Asia)
- Regional supplier governance owned by regions – not aligned globally
- Varied levels of supply base maturity
- Culture and language barriers
Global Supplier Quality Manual
In developing the solution to the concerns mentioned above, the exercise started with:
- Consolidating control under a centralized supplier development function
- Moving to “center-led” governance for policies, procedures and standards development.
The Global Supplier Quality Manual was developed to communicate the high-level expectations to each global supplier in a consistent manner. Agreeing to the execution of the Global Supplier Quality Manual became part of a supplier’s contractual agreement.
Success!

Suppliers were able to gain clarity from the Global Supplier Quality Manual:
- Expectations were established from the up-front auditing process
- Discipline was maintained from onsite assessments and site-level audits.
- Continuity was assured through reassessments (once every three years
- Self-Assessment was possible once scores were confirmed
- Continuous Improvement came into effect as the client continued to meet with key suppliers to discuss the business requirements and improve and tweak those requirements as desired.
Benefits
The benefits of developing and deploying the Global Supplier Quality Manual were evident both to the manufacturer and the global suppliers almost immediately!
- Clarification of customer expectations to it’s supply base
- Establishing a set of standards for suppliers to use
- Eliminating ambiguity and process waste for both customer and supplier
- Establishing alignment to internal policies, procedures and standards
- Reducing supplier product development times
- Improving supplier launch readiness
- Reducing supplier PCA lead-times
- Cost reductions driven by improved supplier quality performance were among some of the benefits. These benefits led to an overall performance improvement in APQP, PPAP, launch discipline, incoming quality and cost.
Would you like to discuss how implementing a Global Supplier Quality Manual can help you and your customers? Contact us today!








 Why Sandalwood?
Why Sandalwood?


 We are a one-stop-shop for launching job rotation for any employer from conception to implementation. Our experts tailor our services to meet the needs of our customers by collaborating with them throughout the entire process. We do not offer cookie cutter solutions for job rotation because the needs of employers vary significantly.
We are a one-stop-shop for launching job rotation for any employer from conception to implementation. Our experts tailor our services to meet the needs of our customers by collaborating with them throughout the entire process. We do not offer cookie cutter solutions for job rotation because the needs of employers vary significantly. Why Sandalwood?
Why Sandalwood?


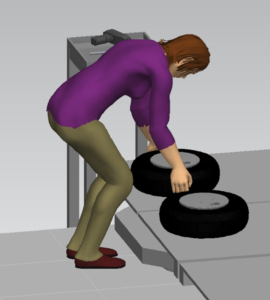
 Sandalwood is pleased to offer solutions above and beyond the traditional ergonomic assessments. With an in-depth knowledge of various digital human modelling software suites, integration and adoption to your health and safety programs has never been easier. Sandalwood is experienced in ergonomic program design as well as industry leaders in digital human modelling services. We have a diverse team that is able the leverage the results from the digital human model to provide in depth risk assessments of future designs and current state. Sandalwood is also able to pair these assessments with expertise and provide guidance on the best solution for you. Sandalwood is also on the forefront of emerging technologies and able to integrate Motion capture, Wearables, and extended or virtual reality into your ergonomic program.
Sandalwood is pleased to offer solutions above and beyond the traditional ergonomic assessments. With an in-depth knowledge of various digital human modelling software suites, integration and adoption to your health and safety programs has never been easier. Sandalwood is experienced in ergonomic program design as well as industry leaders in digital human modelling services. We have a diverse team that is able the leverage the results from the digital human model to provide in depth risk assessments of future designs and current state. Sandalwood is also able to pair these assessments with expertise and provide guidance on the best solution for you. Sandalwood is also on the forefront of emerging technologies and able to integrate Motion capture, Wearables, and extended or virtual reality into your ergonomic program.